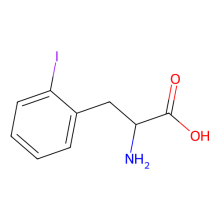
ncAA Structure (png, jpg, jpeg)
ncAA Foundational Publication Support
Tharp, Jeffery M, Yane-Shih Wang, Yan-Jiun Lee, Yanyan Yang, and Wenshe R Liu. (2014) 2014. “Genetic Incorporation Of Seven Ortho-Substituted Phenylalanine Derivatives.”. Acs Chemical Biology 9 (4): 884-90. doi:10.1021/cb400917a.
ncAA Utility
of potential use for protein labeling via Suzuki-Miyaura cross coupling reaction
ncAA Source
Exogenous - Purchased
ncAA Availability
Can be purchased https://www.sigmaaldrich.com/US/en/product/achemblock/advh9873337f?context=bbe
RS/tRNA Pair Usage Information
Yielded 20 mg/L GFPTAG2 when at 2 mM in E. coli using GMML media. Fidelity confirmed by MS. In LB media yielded 32 mg/L (~2 times that obtained with Phe) of GFPTAG27. No MS done. Yielded 4.3 fold increase in fluorescence using HEK293T cells to produce an EGFR-GFPTAG128 when at 5 mM. No MS done.
ncAA Synonyms
2-Iodo-l-phenylalanine
167817-55-2
(S)-2-Amino-3-(2-iodophenyl)propanoic acid
L-Phenylalanine, 2-iodo-
(2S)-2-AMINO-3-(2-IODOPHENYL)PROPANOIC ACID
H-Phe(2-I)-OH
2-iodo-phenylalanine
MFCD01860665
(S)-2-Amino-3-(2-iodophenyl)propanoicacid
SCHEMBL158930
BKXVGLPBXYBDDM-QMMMGPOBSA-N
167817-55-2
(S)-2-Amino-3-(2-iodophenyl)propanoic acid
L-Phenylalanine, 2-iodo-
(2S)-2-AMINO-3-(2-IODOPHENYL)PROPANOIC ACID
H-Phe(2-I)-OH
2-iodo-phenylalanine
MFCD01860665
(S)-2-Amino-3-(2-iodophenyl)propanoicacid
SCHEMBL158930
BKXVGLPBXYBDDM-QMMMGPOBSA-N
ChEBI ID
233124
PubChem Link