ncAA Structure (png, jpg, jpeg)
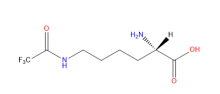
ncAA Foundational Publication Support
Zhang, F., Q. Zhou, G. Yang, L. An, F. Li, and J. Wang. n.d. “A Genetically Encoded 19F Nmr Probe For Lysine Acetylation” 54: 3879+.
ncAA Usage Publications
Hill, Lindsay K., Joseph A. Frezzo, Priya Katyal, Dung Minh Hoang, Zakia Ben Youss Gironda, Cynthia Xu, Xuan Xie, Erika Delgado-Fukushima, Youssef Z. Wadghiri, and Jin Kim Montclare. (mar) 2019. “Protein-Engineered Nanoscale Micelles For Dynamic 19F Magnetic Resonance And Therapeutic Drug Delivery”. Acs Nano 13: 2969-2985. doi:10.1021/acsnano.8b07481.
ncAA Utility
They are used for sites specifically studying post-translational modification acetylation events on particular lysine residues. Also be used as an F19 NMR probe.
ncAA Source
Exogenous - Purchased
ncAA Availability
Can be purchased from https://www.sigmaaldrich.com/US/en/product/sigma/53604
RS/tRNA Pair Usage Information
AcKRS2 has better incorporation efficiency of TFAcK at 5mM in sfGFP in E.coli compared to TFAcKRS. USE AcKRS2 for TFAcK incorporation.
ncAA Synonyms
H-Lys(Tfa)-OH
N6-Trifluoroacetyl-L-lysine
N-6-Trifluoroacetyl-L-lysine
N6-(Trifluoroacetyl)-L-lysine
Nepsilon-Trifluoroacetyl-L-lysine
N6-Trifluoroaccety-L-lysine
N(6)-trifluoroacetyl-L-lysine
(2S)-2-amino-6-[(2,2,2-trifluoroacetyl)amino]hexanoic acid
L-Lysine, N6-(2,2,2-trifluoroacetyl)-
L-Lysine, N6-(trifluoroacetyl)-
(S)-2-Amino-6-(2,2,2-trifluoroacetamido)hexanoic acid
CHEBI:61064
N(6)-(trifluoroacetyl)-L-lysine
TfAcK
n-epsilon-trifluoroacetyl-l-lysine
epsilon-TFA-lysine
N6-(2,2,2-Trifluoroacetyl)-L-lysine; N6-(Trifluoroacetyl)lysine; Nepsilon-Trifluoroacetyl-L-lysine; N?-(Trifluoroacetyl)-L-lysine;
Lysine, N6-(trifluoroacetyl)-, L-
trifluoroacetyl lysine
N6-Trifluoroacetyl-L-lysine
N-6-Trifluoroacetyl-L-lysine
N6-(Trifluoroacetyl)-L-lysine
Nepsilon-Trifluoroacetyl-L-lysine
N6-Trifluoroaccety-L-lysine
N(6)-trifluoroacetyl-L-lysine
(2S)-2-amino-6-[(2,2,2-trifluoroacetyl)amino]hexanoic acid
L-Lysine, N6-(2,2,2-trifluoroacetyl)-
L-Lysine, N6-(trifluoroacetyl)-
(S)-2-Amino-6-(2,2,2-trifluoroacetamido)hexanoic acid
CHEBI:61064
N(6)-(trifluoroacetyl)-L-lysine
TfAcK
n-epsilon-trifluoroacetyl-l-lysine
epsilon-TFA-lysine
N6-(2,2,2-Trifluoroacetyl)-L-lysine; N6-(Trifluoroacetyl)lysine; Nepsilon-Trifluoroacetyl-L-lysine; N?-(Trifluoroacetyl)-L-lysine;
Lysine, N6-(trifluoroacetyl)-, L-
trifluoroacetyl lysine
ChEBI ID
61064
ncAA Additional Notes
Unstable, over time degrades in the Trifluoroacetic acid.
