RS/tRNA Foundational Publication Support
Wang, Yane-Shih, Xinqiang Fang, Ashley L Wallace, Bo Wu, and Wenshe R Liu. (2012) 2012. “A Rationally Designed Pyrrolysyl-Trna Synthetase Mutant With A Broad Substrate Spectrum.”. Journal Of The American Chemical Society 134 (6): 2950-3. doi:10.1021/ja211972x.
Wang, Yane-Shih, Xinqiang Fang, Hsueh-Ying Chen, Bo Wu, Zhiyong U Wang, Christian Hilty, and Wenshe R Liu. (2013) 2013. “Genetic Incorporation Of Twelve Meta-Substituted Phenylalanine Derivatives Using A Single Pyrrolysyl-Trna Synthetase Mutant.”. Acs Chemical Biology 8 (2): 405-15. doi:10.1021/cb300512r.
Tharp, Jeffery M, Yane-Shih Wang, Yan-Jiun Lee, Yanyan Yang, and Wenshe R Liu. (2014) 2014. “Genetic Incorporation Of Seven Ortho-Substituted Phenylalanine Derivatives.”. Acs Chemical Biology 9 (4): 884-90. doi:10.1021/cb400917a.
Wang, Yane-Shih, William K Russell, Zhiyong Wang, Wei Wan, Lindsey E Dodd, Pei-Jing Pai, David H Russell, and Wenshe R Liu. (2011) 2011. “The De Novo Engineering Of Pyrrolysyl-Trna Synthetase For Genetic Incorporation Of L-Phenylalanine And Its Derivatives.”. Molecular Biosystems 7 (3): 714-7. doi:10.1039/c0mb00217h.
RS/tRNA Pair Development Year
2012
ncAA(s) Incorporated
2-iodo-L-phenylalanine
ncAA Structure (png, jpg, jpeg)
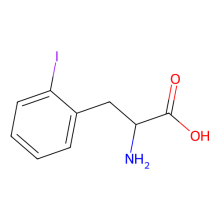
ncAA Utility
of potential use for protein labeling via Suzuki-Miyaura cross coupling reaction
2-chloro-L-phenylalanine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
n/a
2-bromo-L-phenylalanine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
n/a
2-methoxy-L-phenylalanine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
na
2-Cyano-L-phenylalanine
ncAA Structure (png, jpg, jpeg)
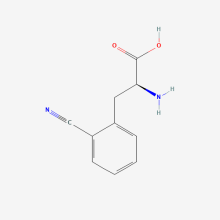
ncAA Utility
Potentially useful as fluorescence-based environmental sensor to study protein conformation. Also, can be an IR probe.
O-allyl-L-tyrosine
ncAA Structure (png, jpg, jpeg)
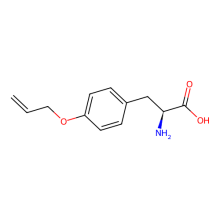
ncAA Utility
in E. coli expressed sfGFP2TAG at 10 mg/L using GMML media
O-methyl-L-tyrosine
ncAA Structure (png, jpg, jpeg)
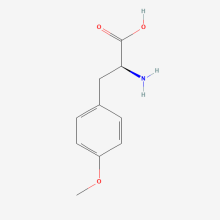
ncAA Utility
Labelling of muscles
(global)
(global)
O-Benzyl-L-tyrosine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
n/a
O-allyl-L-tyrosine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
in E. coli expressed sfGFP2TAG at 10 mg/L using GMML media
O-tert-butyl-L-tyrosine (Tby)
ncAA Structure (png, jpg, jpeg)

ncAA Utility
NMR tag for proteins
O-propargyl-L-tyrosine
ncAA Structure (png, jpg, jpeg)
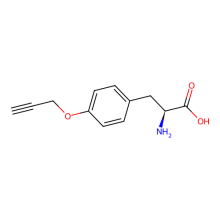
ncAA Utility
Reactive handle for azide-alkyne cycloaddition
O-3-butenyl-L-tyrosine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
n/a
O-4-pentenyl-L-tyrosine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
n/a
3-chloro-L-phenylalanine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
n/a
3-bromo-L-phenylalanine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
n/a
3-iodo-L-phenylalanine
ncAA Structure (png, jpg, jpeg)
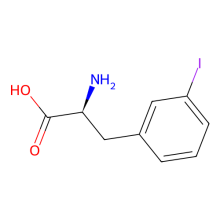
ncAA Utility
can be used for protein ligation using Suzuki-Miyaura cross coupling reaction
3-methyl-L-phenylalanine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
n/a
3-methoxy-L-phenylalanine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
n/a
3-trifluoromethyl-L-phenylalanine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
Useful as an NMR probe, and is an alternative to the para-CF3-Phe ncAA.
3-cyano-L-phenylalanine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
can be a useful IR probe
3-nitro-L-phenylalanine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
can quench Trp fluoresence via FRET in a distance dependent manner. May also be useful as a control for use in studies of the roles of 3-nitroTyr in protein structure-function.
3-acetyl-L-phenylalanine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
can be used for protein ligation
3-allyl-L-phenylalanine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
useful for protein ligations
3-azido-L-phenylalanine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
useful for protein ligations
3-Formyl-L phenylalanine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
Reactive handle for reaction with hydroxylamine dyes
RS Organism of Origin
Parent RS
RS Mutations
N346A
C348A
C348A
tRNA Organism of Origin
Parent tRNA
tRNA Anticodon
CUA
RS/tRNA Availability
Addgene 127411 (called pEVOL-pylT-N346A/C348A) is this RS in a plasmid for use in E. coli.
Addgene 127445 (called pEDF-PhdRS) is this RS in a plasmid for use in to M13 bacteriophage for phage display based selections
Addgene 127445 (called pEDF-PhdRS) is this RS in a plasmid for use in to M13 bacteriophage for phage display based selections
RS/tRNA Additional Notes
Also referred to as PhdRS. This RS can incorporate over 40 mono-substituted phenylalanine derivatives and tyrosinyl ethers. It has as notable background incorporation of Phe, but can nevertheless show high fidelity in a minimal media (i.e. ncAA incorporation outcompetes the Phe present). Fidelity was not assessed in rich media.
2012 paper shows incorporation of some para-Phe (i.e. 4-Phe) derivatives. The 6 best incorporated are all O-alkyl derivatives, so are more simply described as Tyr derivatives. Using sfGFP2TAG and 2 mM ncAA in GMML media, larger substituent ncAAs expressed 1 to 7 times better than with 2 mM Phe, the best yielding ~10 mg/L. ncAAs with smaller para-substituents (Cl, Br, I, NO2, NH2, CN, N3) were incorporated, but all at lower levels than even those obtained with Phe present, and so they are not listed here.
2013 paper shows many meta-Phe (i.e. 3-Phe) derivatives (Cl, Br, I, Me, CF3, OMe, CN, NO2, Acetyl, allyl, azido) incorporate better than the para-Phe derivatives, yielding 5-40 mg/L of sfGFP2TAG in GMML media supplemented with 2 mM L-enantiomer ncAA, with fidelity shown by MS analysis. Meta-F-Phe was toxic and incorporated poorly; meta-NH2-Phe did not incorporate. Production of sfGFP27TAG incorporating m-CF3-Phe was ~80 mg/L; fidelity was not assessed by MS.
2014 paper shows incorporation of ortho-Phe (i.e. 2-Phe) derivatives: Cl, Br, I, Me, MeO, NO2 and CN. Using sfGFP2TAG and 2 mM ncAA, all expressed reasonably (24-48 mg/L) in GMML media with MS showing high fidelity. 2-CF3-Phe and 2-F-Phe incorporated poorly; the former had more Phe than 2-CF3-Phe, and the latter produced a mix of many proteins appearing to have F-Phe incorporated in the place of Phe. In LB media and using sfGFP27TAG, all but 2-MeO-Phe expressed well but no MS fidelity check was done. 2-MeO-Phe led to poor expression and MS showed protein had a 50/50 mix of Phe and 2MeO-Phe. Using EGFR-GFP128TAG, trials in HEK293T cells with 5 mM ncAA showed ncAA-dependent increases in fluorescence for the Cl, I, Me, MeO, NO2 and CN derivatives; the Br derivative killed the cells.
2012 paper shows incorporation of some para-Phe (i.e. 4-Phe) derivatives. The 6 best incorporated are all O-alkyl derivatives, so are more simply described as Tyr derivatives. Using sfGFP2TAG and 2 mM ncAA in GMML media, larger substituent ncAAs expressed 1 to 7 times better than with 2 mM Phe, the best yielding ~10 mg/L. ncAAs with smaller para-substituents (Cl, Br, I, NO2, NH2, CN, N3) were incorporated, but all at lower levels than even those obtained with Phe present, and so they are not listed here.
2013 paper shows many meta-Phe (i.e. 3-Phe) derivatives (Cl, Br, I, Me, CF3, OMe, CN, NO2, Acetyl, allyl, azido) incorporate better than the para-Phe derivatives, yielding 5-40 mg/L of sfGFP2TAG in GMML media supplemented with 2 mM L-enantiomer ncAA, with fidelity shown by MS analysis. Meta-F-Phe was toxic and incorporated poorly; meta-NH2-Phe did not incorporate. Production of sfGFP27TAG incorporating m-CF3-Phe was ~80 mg/L; fidelity was not assessed by MS.
2014 paper shows incorporation of ortho-Phe (i.e. 2-Phe) derivatives: Cl, Br, I, Me, MeO, NO2 and CN. Using sfGFP2TAG and 2 mM ncAA, all expressed reasonably (24-48 mg/L) in GMML media with MS showing high fidelity. 2-CF3-Phe and 2-F-Phe incorporated poorly; the former had more Phe than 2-CF3-Phe, and the latter produced a mix of many proteins appearing to have F-Phe incorporated in the place of Phe. In LB media and using sfGFP27TAG, all but 2-MeO-Phe expressed well but no MS fidelity check was done. 2-MeO-Phe led to poor expression and MS showed protein had a 50/50 mix of Phe and 2MeO-Phe. Using EGFR-GFP128TAG, trials in HEK293T cells with 5 mM ncAA showed ncAA-dependent increases in fluorescence for the Cl, I, Me, MeO, NO2 and CN derivatives; the Br derivative killed the cells.
