ncAA Structure (png, jpg, jpeg)
ncAA Foundational Publication Support
Santoro, Stephen W, Lei Wang, Brad Herberich, David S King, and Peter G Schultz. (2002) 2002. “An Efficient System For The Evolution Of Aminoacyl-Trna Synthetase Specificity.”. Nature Biotechnology 20 (10): 1044-8.
Young, Douglas D, Travis S Young, Michael Jahnz, Insha Ahmad, Glen Spraggon, and Peter G Schultz. (2011) 2011. “An Evolved Aminoacyl-Trna Synthetase With Atypical Polysubstrate Specificity.”. Biochemistry 50 (11): 1894-900. doi:10.1021/bi101929e.
ncAA Utility
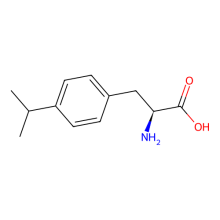
tyrosine analog
ncAA Source
Endogenous
ncAA Availability
can be brought from https://www.sigmaaldrich.com/US/en/product/achemblock/advh0430a373?context=bbe
RS/tRNA Pair Usage Information
need to replace dummy RS with pIF RS from 2002 foundational paper
ncAA Synonyms
(S)-2-AMINO-3-(4-ISOPROPYL-PHENYL)PROPIONIC ACID
L-Phenylalanine,4-(1-methylethyl)-
(S)-2-AMINO-3-(4-ISOPROPYLPHENYL)PROPANOIC ACID
(2S)-2-amino-3-(4-propan-2-ylphenyl)propanoic acid
L-Phenylalanine,4-(1-methylethyl)-
(S)-2-AMINO-3-(4-ISOPROPYLPHENYL)PROPANOIC ACID
(2S)-2-amino-3-(4-propan-2-ylphenyl)propanoic acid
ChEBI ID
232656
PubChem Link
