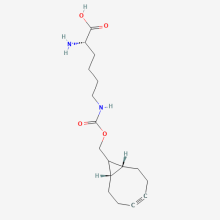
ncAA Structure (png, jpg, jpeg)
ncAA Foundational Publication Support
Lang, Kathrin, Lloyd Davis, Stephen Wallace, Mohan Mahesh, Daniel J Cox, Melissa L Blackman, Joseph M Fox, and Jason W Chin. (2012) 2012. “Genetic Encoding Of Bicyclononynes And Trans-Cyclooctenes For Site-Specific Protein Labeling In Vitro And In Live Mammalian Cells Via Rapid Fluorogenic Diels-Alder Reactions.”. Journal Of The American Chemical Society 134 (25): 10317-20. doi:10.1021/ja302832g.
Nikić, Ivana, Jun Hee Kang, Gemma Estrada Girona, Iker Valle Aramburu, and Edward A Lemke. (2015) 2015. “Labeling Proteins On Live Mammalian Cells Using Click Chemistry.”. Nature Protocols 10 (5): 780-91. doi:10.1038/nprot.2015.045.
ncAA Protocols
Nikić, Ivana, Jun Hee Kang, Gemma Estrada Girona, Iker Valle Aramburu, and Edward A Lemke. (2015) 2015. “Labeling Proteins On Live Mammalian Cells Using Click Chemistry.”. Nature Protocols 10 (5): 780-91. doi:10.1038/nprot.2015.045.
ncAA Utility
Reactive handle for SPAAC
ncAA Source
Exogenous - Synthesized
ncAA Availability
endoBCN is SiChem catalog number SC8016
exoBCN is SiChem catalog 8014
racemicBCN is SiChem catalog number SC8003
exoBCN is SiChem catalog 8014
racemicBCN is SiChem catalog number SC8003
RS/tRNA Pair Usage Information
No head to head comparison of the RSs is available, but the MmPylRS-AF is available from Addgene and it has been showing to work with both exoBCN and endoBCN. The BCNRS is based on the MbPylRS and both RSs have been shown to work in E coli and mammalian cells.
ncAA Synonyms
(Rac)-BCN-L-Lysine
N6-({[(1R,8S,9s)-bicyclo[6.1.0]non-4-yn-9-yl]methoxy}carbonyl)-L-lysine
(2S)-2-Amino-6-((((1R,8S)-bicyclo[6.1.0]non-4-yn-9-ylmethoxy)carbonyl)amino)hexanoic acid
(S)-2-Amino-6-((((1R,8S,9s)-bicyclo[6.1.0]non-4-yn-9-ylmethoxy)carbonyl)amino)hexanoic acid
(2S)-2-Amino-6-({[(1R,8S)-bicyclo[6.1.0]non-4-yn-9-ylmethoxy]carbonyl}amino)hexanoic acid
bicyclo[6.1.0]nonyne-lysine
N6-({[(1R,8S,9s)-bicyclo[6.1.0]non-4-yn-9-yl]methoxy}carbonyl)-L-lysine
(2S)-2-Amino-6-((((1R,8S)-bicyclo[6.1.0]non-4-yn-9-ylmethoxy)carbonyl)amino)hexanoic acid
(S)-2-Amino-6-((((1R,8S,9s)-bicyclo[6.1.0]non-4-yn-9-ylmethoxy)carbonyl)amino)hexanoic acid
(2S)-2-Amino-6-({[(1R,8S)-bicyclo[6.1.0]non-4-yn-9-ylmethoxy]carbonyl}amino)hexanoic acid
bicyclo[6.1.0]nonyne-lysine
ChEBI ID
233066
PubChem Link
