RS/tRNA Foundational Publication Support
Amiram, Miriam, Adrian D. Haimovich, Chenguang Fan, Yane-Shih Wang, Hans-Rudolf Aerni, Ioanna Ntai, Daniel W. Moonan, et al. (dec) 2015. “Evolution Of Translation Machinery In Recoded Bacteria Enables Multi-Site Incorporation Of Nonstandard Amino Acids”. Nature Biotechnology 33: 1272-1279. doi:10.1038/nbt.3372.
Wang, Lei, Zhiwen Zhang, Ansgar Brock, and Peter G Schultz. (2003) 2003. “Addition Of The Keto Functional Group To The Genetic Code Of Escherichia Coli.”. Proceedings Of The National Academy Of Sciences Of The United States Of America 100 (1): 56-61.
Young, Travis S, Insha Ahmad, Jun A Yin, and Peter G Schultz. (2010) 2010. “An Enhanced System For Unnatural Amino Acid Mutagenesis In E. Coli.”. Journal Of Molecular Biology 395 (2): 361-74. doi:10.1016/j.jmb.2009.10.030.
Young, Douglas D, Travis S Young, Michael Jahnz, Insha Ahmad, Glen Spraggon, and Peter G Schultz. (2011) 2011. “An Evolved Aminoacyl-Trna Synthetase With Atypical Polysubstrate Specificity.”. Biochemistry 50 (11): 1894-900. doi:10.1021/bi101929e.
RS/tRNA Pair Development Year
2015
ncAA(s) Incorporated
p-acetyl-L-phenylalanine
ncAA Structure (png, jpg, jpeg)
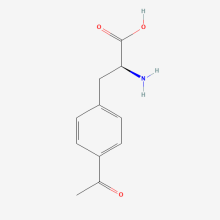
ncAA Utility
Site-directed spin labeling, electron paramagnetic resonance
p-azido-L-phenylalanine (pAzF)
ncAA Structure (png, jpg, jpeg)
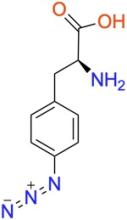
ncAA Utility
Used as a photocrosslinker, allowing for crosslinking and bioorthogonal click-chemistry ligation of proteins via strain-promoted cycloadditions (SPAAC) with suitably functionalized molecules.
p-iodo-L-phenylalanine
ncAA Structure (png, jpg, jpeg)
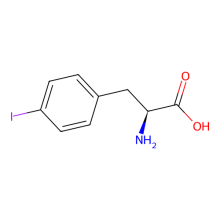
ncAA Utility
Can be used for phasing in protein crystallography.
O-methyl-L-tyrosine
ncAA Structure (png, jpg, jpeg)
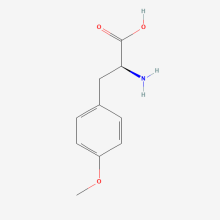
ncAA Utility
Labelling of muscles
(global)
(global)
RS Organism of Origin
Parent RS
RS Mutations
Y32L
D158G
I159C
L162R
A167D
R257G
D158G
I159C
L162R
A167D
R257G
tRNA Organism of Origin
Parent tRNA
tRNA Anticodon
CUA
Other tRNA Mutations
C17A
U17aG
U20C
G37A
U47G
U17aG
U20C
G37A
U47G
RS/tRNA Availability
Addgene plasmid #73545
Used in what cell line?
RS/tRNA Additional Notes
This RS/tRNA pair was evolved in the C321.ΔA E. coli strain from the AcetylPhe RS (pAcF-RS) originally reported in 2002 foundational paper, used as a model RS in the 2010 foundational paper and characterized for permissivity in the 2011 foundational paper. In 2015, further residues were allowed to evolve to optimize p-acetyl-Phe and tRNA interactions and improve inserting multiple ncAAs into a given protein using low RS levels. This RS showed 17-fold increased expression of GFP-3sites compared to pAcFRS using a chromosome incorporated RS. Figure 4a of foundational paper shows that when included as multicopy plasmid, it still highly outperforms the starting RS for incorporating 30 ncAAs in an ELP(Elastin-Like-Protein)-GFP construct, but does not outperform it when expressing GFP with 3 ncAAs, and also has a much lower fidelity (~75% as much protein production in the absence of ncAA). This RS was also shown to be especially effective at incorporating p-azidoPhe, p-IodoPhe, and O-methylTyr.
