ncAA Structure (png, jpg, jpeg)
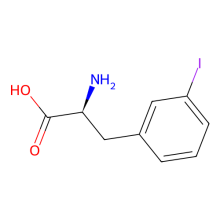
ncAA Foundational Publication Support
Wang, Yane-Shih, Xinqiang Fang, Hsueh-Ying Chen, Bo Wu, Zhiyong U Wang, Christian Hilty, and Wenshe R Liu. (2013) 2013. “Genetic Incorporation Of Twelve Meta-Substituted Phenylalanine Derivatives Using A Single Pyrrolysyl-Trna Synthetase Mutant.”. Acs Chemical Biology 8 (2): 405-15. doi:10.1021/cb300512r.
ncAA Utility
can be used for protein ligation using Suzuki-Miyaura cross coupling reaction
ncAA Source
Exogenous - Purchased
ncAA Availability
Can be purchased from https://www.scbt.com/p/3-iodo-l-phenylalanine-20846-39-3
RS/tRNA Pair Usage Information
incorporated at high levels (20 mg/L) with fidelity in E coli grown with 2 mM ncAA in GMML media
ncAA Synonyms
3-Iodo-L-Phenylalanine
20846-39-3
(S)-2-Amino-3-(3-iodophenyl)propanoic acid
L-Phenylalanine, 3-iodo-
(2S)-2-AMINO-3-(3-IODOPHENYL)PROPANOIC ACID
DTXSID00587780
(S)-2-Amino-3-(3-iodophenyl)propanoicacid
H-Phe(3-I)-OH
3-iodophenylalanine
L-3-Iodophe
MFCD01860664
20846-39-3
(S)-2-Amino-3-(3-iodophenyl)propanoic acid
L-Phenylalanine, 3-iodo-
(2S)-2-AMINO-3-(3-IODOPHENYL)PROPANOIC ACID
DTXSID00587780
(S)-2-Amino-3-(3-iodophenyl)propanoicacid
H-Phe(3-I)-OH
3-iodophenylalanine
L-3-Iodophe
MFCD01860664
ChEBI ID
233132
PubChem Link
