ncAA Structure (png, jpg, jpeg)
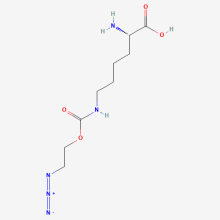
ncAA Foundational Publication Support
Li, Yiming, Man Pan, Yitong Li, Yichao Huang, and Qingxiang Guo. (2013) 2013. “Thiol-Yne Radical Reaction Mediated Site-Specific Protein Labeling Via Genetic Incorporation Of An Alkynyl-L-Lysine Analogue.”. Organic & Biomolecular Chemistry 11 (16): 2624-9. doi:10.1039/c3ob27116a.
ncAA Utility
Reactive handle for azide-alkyne cycloaddition
ncAA Source
Exogenous - Synthesized
ncAA Availability
n/a
ncAA Synonyms
(2S)-2-amino-6-{[(2-azidoethoxy)carbonyl]amino}hexanoic acid
1167421-25-1
(S)-2-Amino-6-(((2-azidoethoxy)carbonyl)amino)hexanoic acid
N?-2-Azidoethyloxycarbonyl-L-lysine
N6-[(2-Azidoethoxy)carbonyl]-L-lysine; (2S)-2-Amino-6-[[(2-azidoethoxy)carbonyl]amino]hexanoic Acid;(S)-2-Amino-6-[(2-azidoethoxy)carbonylamino]hexanoic Acid;N6-[(2-Azidoethoxy)carbonyl]-L-lysine;
SCHEMBL15546760
AKOS030212928
N6-((2-azidoethoxy)carbonyl)-L-lysine
NEpsilon-2-Azidoethyloxycarbonyl-L-lysine
1167421-25-1
(S)-2-Amino-6-(((2-azidoethoxy)carbonyl)amino)hexanoic acid
N?-2-Azidoethyloxycarbonyl-L-lysine
N6-[(2-Azidoethoxy)carbonyl]-L-lysine; (2S)-2-Amino-6-[[(2-azidoethoxy)carbonyl]amino]hexanoic Acid;(S)-2-Amino-6-[(2-azidoethoxy)carbonylamino]hexanoic Acid;N6-[(2-Azidoethoxy)carbonyl]-L-lysine;
SCHEMBL15546760
AKOS030212928
N6-((2-azidoethoxy)carbonyl)-L-lysine
NEpsilon-2-Azidoethyloxycarbonyl-L-lysine
ChEBI ID
233053
PubChem Link
