RS/tRNA Foundational Publication Support
Neumann, Heinz, Sew Y Peak-Chew, and Jason W Chin. (2008) 2008. “Genetically Encoding N(Epsilon)-Acetyllysine In Recombinant Proteins.”. Nature Chemical Biology 4 (4): 232-4. doi:10.1038/nchembio.73.
RS/tRNA Usage Publications
Sun, Yanan, Yanchi Chen, Yaxin Xu, Yuqing Zhang, Minghao Lu, Manjia Li, Liyan Zhou, and Tao Peng. (jul) 2022. “Genetic Encoding Of Ε-N-L-Lactyllysine For Detecting Delactylase Activity In Living Cells.”. Chemical Communications (Cambridge, England) 58: 8544-8547. doi:10.1039/d2cc02643k.
RS/tRNA Pair Development Year
2008
ncAA(s) Incorporated
Nε-acetyl-L-lysine
ncAA Structure (png, jpg, jpeg)
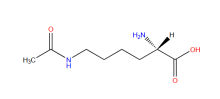
ncAA Utility
They are used for sites specifically studying post-translational modification acetylation events on particular lysine residues.
N6-Trifluoroacetyl-L-lysine
ncAA Structure (png, jpg, jpeg)
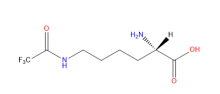
ncAA Utility
They are used for sites specifically studying post-translational modification acetylation events on particular lysine residues. Also be used as an F19 NMR probe.
RS Organism of Origin
Parent RS
RS Mutations
L270I
Y271L
L274A
C313F
Y271L
L274A
C313F
tRNA Organism of Origin
Parent tRNA
tRNA Anticodon
CUA
Other tRNA Mutations
N/A
Multiple tRNAs?
N/A
RS/tRNA Availability
It is not Commercially available but can be made by applying the mutation to Mb-PylRS/tRNA.
RS/tRNA Additional Notes
In the original publication, nothing was reported about the efficiency or fidelity of this RS. Permissive for TfAcK. It is shown to have the lowest incorporation of AcK at 5mM compared to AckRS1 and AcKRS3 in sfGFP in E.coli, when measuring fluorescence. It however has the highest incorporation for TfAcK among them when compared to MbTfAcKRS at 5mM TfAcK in E.coli.
