RS/tRNA Foundational Publication Support
Furman, Jennifer L, Mingchao Kang, Seihyun Choi, Yu Cao, Erik D Wold, Sophie B Sun, Vaughn Smider V, Peter G Schultz, and Chan Hyuk Kim. (2014) 2014. “A Genetically Encoded Aza-Michael Acceptor For Covalent Cross-Linking Of Protein-Receptor Complexes.”. Journal Of The American Chemical Society 136 (23): 8411-7. doi:10.1021/ja502851h.
RS/tRNA Pair Development Year
2014
ncAA(s) Incorporated
Nε-Acryloyl-L-lysine
ncAA Structure (png, jpg, jpeg)
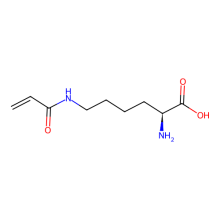
ncAA Utility
Reactive handle for 1,4-‐cycloadditions, radical copolymerisation and 1,3-‐dipolar cycloaddition. Also used as an reactive handle for ‘photo-‐click’
RS Organism of Origin
Parent RS
RS Mutations
L270I
L274A
C313F
T349F
L274A
C313F
T349F
tRNA Organism of Origin
Parent tRNA
tRNA Anticodon
CUA
RS/tRNA Availability
N/A
Used in what cell line?
RS/tRNA Additional Notes
In fluorescence based assays, GFP with an Asn149TAG mutation was co-expressed with 20-fold increase when in the presence of 5mM of Nε-Acryloyl-L-lysine and the AcrKRS.
In E. coli cells, AcrKRS/tRNA pair enabled the expression of Herceptin antigen binding fragment(Fab) mutants in the presence of 5mM Nε-Acryloyl-L-lysine within a TB media, yielding ~1-2mg/L protein. ESI-MS confirmed incorporation of Nε-Acryloyl-L-lysine at all suppression sites(LC-Tyr92, LC-Thr93, HC-Gly103).
In E. coli cells, AcrKRS/tRNA pair enabled the expression of Herceptin antigen binding fragment(Fab) mutants in the presence of 5mM Nε-Acryloyl-L-lysine within a TB media, yielding ~1-2mg/L protein. ESI-MS confirmed incorporation of Nε-Acryloyl-L-lysine at all suppression sites(LC-Tyr92, LC-Thr93, HC-Gly103).
