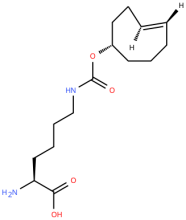
ncAA Structure (png, jpg, jpeg)
ncAA Foundational Publication Support
Lang, Kathrin, Lloyd Davis, Stephen Wallace, Mohan Mahesh, Daniel J Cox, Melissa L Blackman, Joseph M Fox, and Jason W Chin. (2012) 2012. “Genetic Encoding Of Bicyclononynes And Trans-Cyclooctenes For Site-Specific Protein Labeling In Vitro And In Live Mammalian Cells Via Rapid Fluorogenic Diels-Alder Reactions.”. Journal Of The American Chemical Society 134 (25): 10317-20. doi:10.1021/ja302832g.
Peng, Tao, and Howard C Hang. (2016) 2016. “Site-Specific Bioorthogonal Labeling For Fluorescence Imaging Of Intracellular Proteins In Living Cells.”. Journal Of The American Chemical Society 138 (43): 14423-14433.
ncAA Utility
Reactive handle for IEDDA.
ncAA Source
Exogenous - Purchased
ncAA Availability
SiChem catalog #SC8004 is the axial isomer, and #SC8060 is the equatorial isomer
RS/tRNA Pair Usage Information
Peng & Hang (2016) showed the UP50 values for the 4'-axial, and 4'-equitorial TCOK were roughly 800 and 200 microM respectively. They also showed that the MmPylRS-AF synthetase did not incorporate the 4'-TCOK ncAAs.
ncAA Synonyms
trans-cyclooct-4-ene-L-lysine
TCO
TCOK
TCO4/EQ
TCO4/AX
TCO4
TCO
TCOK
TCO4/EQ
TCO4/AX
TCO4
ChEBI ID
233068
ncAA Additional Notes
Peng & Hong (2016) noted the 2'-axial TCOK (a.k.a TCO* or TCO*A) is more stable than the 4' isomer
