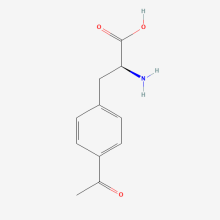
ncAA Structure (png, jpg, jpeg)
ncAA Foundational Publication Support
Fleissner, Mark R, Eric M Brustad, Tamás Kálai, Christian Altenbach, Duilio Cascio, Francis B Peters, Kálmán Hideg, Sebastian Peuker, Peter G Schultz, and Wayne L Hubbell. (2009) 2009. “Site-Directed Spin Labeling Of A Genetically Encoded Unnatural Amino Acid.”. Proceedings Of The National Academy Of Sciences Of The United States Of America 106 (51): 21637-42. doi:10.1073/pnas.0912009106.
Young, Douglas D, Travis S Young, Michael Jahnz, Insha Ahmad, Glen Spraggon, and Peter G Schultz. (2011) 2011. “An Evolved Aminoacyl-Trna Synthetase With Atypical Polysubstrate Specificity.”. Biochemistry 50 (11): 1894-900. doi:10.1021/bi101929e.
Wang, Lei, Zhiwen Zhang, Ansgar Brock, and Peter G Schultz. (2003) 2003. “Addition Of The Keto Functional Group To The Genetic Code Of Escherichia Coli.”. Proceedings Of The National Academy Of Sciences Of The United States Of America 100 (1): 56-61.
ncAA Utility
Site-directed spin labeling, electron paramagnetic resonance
ncAA Source
Exogenous - Purchased
ncAA Availability
Can be purchased from https://pr.vwr.com/store/product/35224843/4-acetyl-l-phenylalanine-hydrochloride-95
ncAA Synonyms
122555-04-8
4-ACETYL-L-PHENYLALANINE
H-Phe(4-Ac)-OH
L-4-Acetylphenylalanine
P-ACETYLPHENYLALANINE
L-Phenylalanine, 4-acetyl-
p-acetyl-L-phenylalanine
(S)-3-(4-Acetylphenyl)-2-aminopropanoic acid
(2S)-3-(4-acetylphenyl)-2-aminopropanoic acid
L-PHE(4-COCH3)
Y61IJN1HNY
4-ACETYL-L-PHENYLALANINE
H-Phe(4-Ac)-OH
L-4-Acetylphenylalanine
P-ACETYLPHENYLALANINE
L-Phenylalanine, 4-acetyl-
p-acetyl-L-phenylalanine
(S)-3-(4-Acetylphenyl)-2-aminopropanoic acid
(2S)-3-(4-acetylphenyl)-2-aminopropanoic acid
L-PHE(4-COCH3)
Y61IJN1HNY
ChEBI ID
40003
