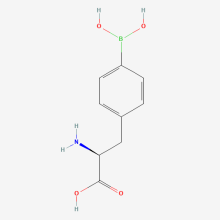
ncAA Structure (png, jpg, jpeg)
ncAA Foundational Publication Support
Chen, Zhi-jie, Wei Ren, Quintin Wright, and Hui-Wang Ai. (2013) 2013. “Genetically Encoded Fluorescent Probe For The Selective Detection Of Peroxynitrite.”. Journal Of The American Chemical Society 135 (40): 14940-3. doi:10.1021/ja408011q.
Brustad, Eric, Mark L Bushey, Jae Wook Lee, Dan Groff, Wenshe Liu, and Peter G Schultz. (2008) 2008. “A Genetically Encoded Boronate-Containing Amino Acid.”. Angewandte Chemie (International Ed. In English) 47 (43): 8220-3. doi:10.1002/anie.200803240.
ncAA Utility
enabled one-step "scarless" protein purification procedure, and can be used to site-specifically label proteins using various boronic acid chemistries. Also, in second foundational paper was used in creating a fluorescent probe for peroxinitrite.
ncAA Source
Exogenous - Purchased
ncAA Availability
Can be purchased from https://www.sigmaaldrich.com/US/en/product/sial/17755
RS/tRNA Pair Usage Information
The B(OH)2PheRS is for use in bacteria.
The p-iodoPhe-RS2 is for use in eukaryotic cells and is available from Addgene
The p-iodoPhe-RS2 is for use in eukaryotic cells and is available from Addgene
ncAA Synonyms
4-Borono-L-phenylalanine
(s)-2-amino-3-(4-boronophenyl)propanoic acid
4-Boronophenylalanine
p-Boronophenylalanine
boronophenylalanine
L-p-boronophenylalanine
(2S)-2-amino-3-(4-boronophenyl)propanoic acid
Borofalan
(s)-2-amino-3-(4-boronophenyl)propanoic acid
4-Boronophenylalanine
p-Boronophenylalanine
boronophenylalanine
L-p-boronophenylalanine
(2S)-2-amino-3-(4-boronophenyl)propanoic acid
Borofalan
ChEBI ID
232826
