RS/tRNA Foundational Publication Support
Schultz, Kathryn C, Lubica Supekova, Youngha Ryu, Jianming Xie, Roshan Perera, and Peter G Schultz. (2006) 2006. “A Genetically Encoded Infrared Probe.”. Journal Of The American Chemical Society 128 (43): 13984-5.
Young, Douglas D, Travis S Young, Michael Jahnz, Insha Ahmad, Glen Spraggon, and Peter G Schultz. (2011) 2011. “An Evolved Aminoacyl-Trna Synthetase With Atypical Polysubstrate Specificity.”. Biochemistry 50 (11): 1894-900. doi:10.1021/bi101929e.
Chatterjee, Abhishek, Sophie B Sun, Jennifer L Furman, Han Xiao, and Peter G Schultz. (2013) 2013. “A Versatile Platform For Single- And Multiple-Unnatural Amino Acid Mutagenesis In Escherichia Coli.”. Biochemistry 52 (10): 1828-37. doi:10.1021/bi4000244.
Wilkinson, Henry C, and Paul A Dalby. (2021) 2021. “Fine-Tuning The Activity And Stability Of An Evolved Enzyme Active-Site Through Noncanonical Amino-Acids.”. The Febs Journal 288 (6): 1935-1955. doi:10.1111/febs.15560.
RS/tRNA Protocols and Structural Information
Lightle, Hailey E, Parmila Kafley, Todd R Lewis, and Rongsheng E Wang. (2023) 2023. “Site-Specific Protein Conjugates Incorporating Para-Azido-L-Phenylalanine For Cellular And In Vivo Imaging.”. Methods (San Diego, Calif.) 219: 95-101. doi:10.1016/j.ymeth.2023.10.001.
RS/tRNA Usage Publications
Chen, Wan-Na, Kekini Kuppan, Michael Lee, Kristaps Jaudzems, Thomas Huber, and Gottfried Otting. (2015) 2015. “O-Tert-Butyltyrosine, An Nmr Tag For High-Molecular-Weight Systems And Measurements Of Submicromolar Ligand Binding Affinities.”. Journal Of The American Chemical Society 137 (13): 4581-6. doi:10.1021/jacs.5b01918.
Loh, Choy Theng, Luke A Adams, Bim Graham, and Gottfried Otting. (2018) 2018. “Genetically Encoded Amino Acids With Tert-Butyl And Trimethylsilyl Groups For Site-Selective Studies Of Proteins By Nmr Spectroscopy.”. Journal Of Biomolecular Nmr 71 (4): 287-293. doi:10.1007/s10858-017-0157-y.
Jabar, Shereen, Luke A Adams, Yao Wang, Luigi Aurelio, Bim Graham, and Gottfried Otting. (2017) 2017. “Chemical Tagging With Tert-Butyl And Trimethylsilyl Groups For Measuring Intermolecular Nuclear Overhauser Effects In A Large Protein-Ligand Complex.”. Chemistry (Weinheim An Der Bergstrasse, Germany) 23 (53): 13033-13036. doi:10.1002/chem.201703531.
Wissler, Haley, Emily Ehlerding, Zhigang Lyu, Yue Zhao, Si Zhang, Anisa Eshraghi, Zakey Buuh, et al. (2019) 2019. “Site-Specific Immuno-Pet Tracer To Image Pd-L1.”. Molecular Pharmaceutics 16 (5): 2028-2036. doi:10.1021/acs.molpharmaceut.9b00010.
Amiram, Miriam, Adrian D. Haimovich, Chenguang Fan, Yane-Shih Wang, Hans-Rudolf Aerni, Ioanna Ntai, Daniel W. Moonan, et al. (dec) 2015. “Evolution Of Translation Machinery In Recoded Bacteria Enables Multi-Site Incorporation Of Nonstandard Amino Acids”. Nature Biotechnology 33: 1272-1279. doi:10.1038/nbt.3372.
RS/tRNA Pair Development Year
2006
ncAA(s) Incorporated
p-cyano-L-phenylalanine
ncAA Structure (png, jpg, jpeg)
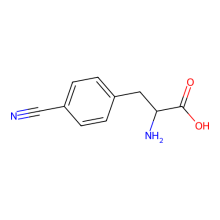
ncAA Utility
FRET Probe (with Trp) and infrared probe
p-azido-L-phenylalanine (pAzF)
ncAA Structure (png, jpg, jpeg)
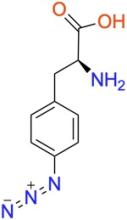
ncAA Utility
Used as a photocrosslinker, allowing for crosslinking and bioorthogonal click-chemistry ligation of proteins via strain-promoted cycloadditions (SPAAC) with suitably functionalized molecules.
p-iodo-L-phenylalanine
ncAA Structure (png, jpg, jpeg)
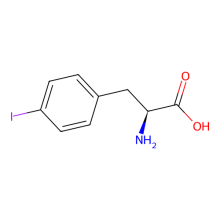
ncAA Utility
Can be used for phasing in protein crystallography.
p-bromo-L-phenylalanine
ncAA Structure (png, jpg, jpeg)
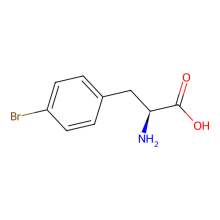
ncAA Utility
Mutant GFP
excitation/ emission
excitation/ emission
p-acetyl-L-phenylalanine
ncAA Structure (png, jpg, jpeg)
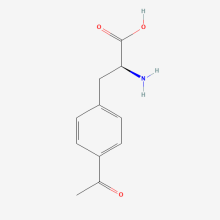
ncAA Utility
Site-directed spin labeling, electron paramagnetic resonance
p-isopropyl-L-phenylalanine
ncAA Structure (png, jpg, jpeg)
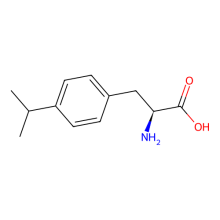
ncAA Utility
tyrosine analog
para-nitro-L-phenylalanine
ncAA Structure (png, jpg, jpeg)

ncAA Utility
n/a
O-allyl-L-tyrosine
ncAA Structure (png, jpg, jpeg)
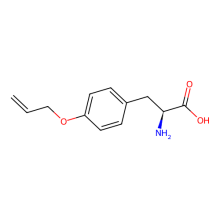
ncAA Utility
in E. coli expressed sfGFP2TAG at 10 mg/L using GMML media
p-ethynyl-L-phenylalanine
ncAA Structure (png, jpg, jpeg)
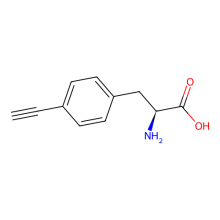
ncAA Utility
FRET Probe
BiphenylAla(BipAla)
ncAA Structure (png, jpg, jpeg)
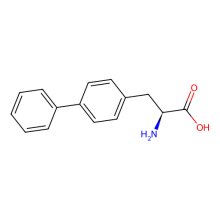
ncAA Utility
Synthetase intermediate developed on way to BpyAla incorporation
O-tert-butyl-L-tyrosine (Tby)
ncAA Structure (png, jpg, jpeg)

ncAA Utility
NMR tag for proteins
O-propargyl-L-tyrosine
ncAA Structure (png, jpg, jpeg)
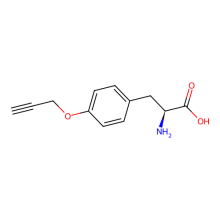
ncAA Utility
Reactive handle for azide-alkyne cycloaddition
p-chloro-L-phenylalanine
ncAA Structure (png, jpg, jpeg)
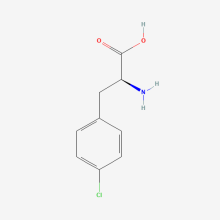
ncAA Utility
NMR probe
p-fluoro-L-phenylalanine
ncAA Structure (png, jpg, jpeg)
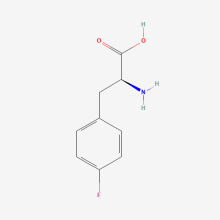
ncAA Utility
NMR probe
p-trifluoroacetyl-L-phenylalanine
ncAA Structure (png, jpg, jpeg)
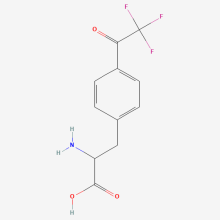
ncAA Utility
Flourescent Probe, Bioorthogonal ligation handle
O-benzyl-L-serine
ncAA Structure (png, jpg, jpeg)
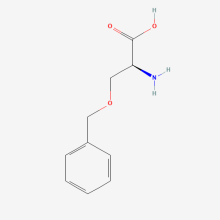
ncAA Utility
n/a
RS Organism of Origin
Parent RS
RS Mutations
Y32L
L65V
F108W
Q109M
D158G
I159A
L65V
F108W
Q109M
D158G
I159A
tRNA Organism of Origin
Parent tRNA
tRNA Anticodon
CUA
Other tRNA Mutations
C17A
U17aG
U20C
G37A
U47G
U17aG
U20C
G37A
U47G
RS/tRNA Availability
pULTRA-CNF plasmid with this RS is available as Addgene Plasmid #48215 (developed in 2013 foundational paper for use in dual encoding)
Used in what cell line?
RS/tRNA Additional Notes
In presence of 2 mM pCNF, MS confirmed full-fidelity incorporation into Z-domain(7). Also installed into myoglobin(64) yielding 30 mg/L, the pCNF provided a useful infrared probe of ligand binding.
2011 foundational paper showed this RS to be unusually permissive (i.e. polyspecific) and identified 18 ncAAs that were all incorporated into myoglobin(107) at between 43 and 96% efficiency (w/ WT expression being ~16 mg/L). Also reported a crystal structure with bound pCNF.
In 2013, putting into pUltra plasmid led to 1.3-2-fold efficiency gains for various ncAAs. And this is the plasmid available from Addgene and is one of the go to systems for installing pAzido-Phe
2021 paper incorporated p-nitro, p-cyano and p-aminoPhe into a transketolase in BL21 and C321.ΔA.exp cells with careful MS analyses indicating both cell lines yielded roughly 80%, 75% and 50% fidelity, respectively.
2011 foundational paper showed this RS to be unusually permissive (i.e. polyspecific) and identified 18 ncAAs that were all incorporated into myoglobin(107) at between 43 and 96% efficiency (w/ WT expression being ~16 mg/L). Also reported a crystal structure with bound pCNF.
In 2013, putting into pUltra plasmid led to 1.3-2-fold efficiency gains for various ncAAs. And this is the plasmid available from Addgene and is one of the go to systems for installing pAzido-Phe
2021 paper incorporated p-nitro, p-cyano and p-aminoPhe into a transketolase in BL21 and C321.ΔA.exp cells with careful MS analyses indicating both cell lines yielded roughly 80%, 75% and 50% fidelity, respectively.
