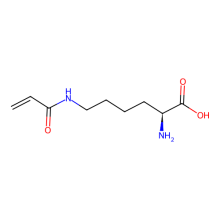
ncAA Structure (png, jpg, jpeg)
ncAA Foundational Publication Support
Lee, Yan-Jiun, Bo Wu, Jeffrey E Raymond, Yu Zeng, Xinqiang Fang, Karen L Wooley, and Wenshe R Liu. (2013) 2013. “A Genetically Encoded Acrylamide Functionality.”. Acs Chemical Biology 8 (8): 1664-70. doi:10.1021/cb400267m.
ncAA Usage Publications
Lee, Yan-Jiun, Bo Wu, Jeffrey E Raymond, Yu Zeng, Xinqiang Fang, Karen L Wooley, and Wenshe R Liu. (2013) 2013. “A Genetically Encoded Acrylamide Functionality.”. Acs Chemical Biology 8 (8): 1664-70. doi:10.1021/cb400267m.
Li, Fahui, Hua Zhang, Yun Sun, Yanchao Pan, Juanzuo Zhou, and Jiangyun Wang. (2013) 2013. “Expanding The Genetic Code For Photoclick Chemistry In E. Coli, Mammalian Cells, And A. Thaliana.”. Angewandte Chemie (International Ed. In English) 52 (37): 9700-4. doi:10.1002/anie.201303477.
ncAA Utility
Reactive handle for 1,4-‐cycloadditions, radical copolymerisation and 1,3-‐dipolar cycloaddition. Also used as an reactive handle for ‘photo-‐click’
ncAA Source
Exogenous - Purchased
ncAA Availability
Can be purchased from https://www.chemimpex.com/products/47693
ncAA Synonyms
48065-82-3
(S)-6-Acrylamido-2-aminohexanoic acid
Nepsilon-acryloyl-L-lysin
MFCD30187477
N?-acryloyl-L-lysin
H-L-Lys(Acryloyl)-OH
(S)-6-Acrylamido-2-aminohexanoic acid
Nepsilon-acryloyl-L-lysin
MFCD30187477
N?-acryloyl-L-lysin
H-L-Lys(Acryloyl)-OH
ChEBI ID
233076
PubChem Link
