ncAA Database
Displaying 26 - 50 of 56 results
| ncAA Name | ncAA Structure | ncAA Utility | Moderation State |
|---|---|---|---|
| Norbornene-methoxycarbonyl-Lysine | 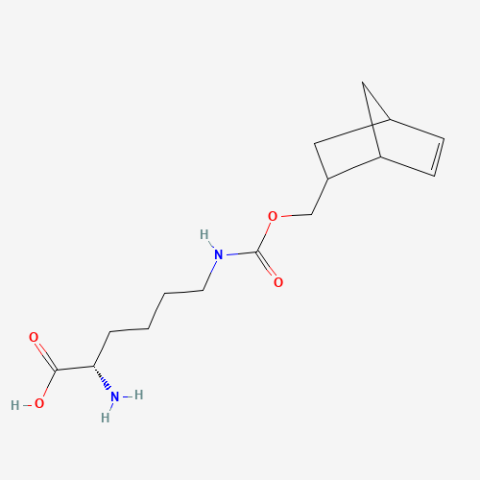
|
Useful for click-chemistry with nitrile imines and tetrazines | Published: Fully Reviewed |
| Norbornene-O–L-Lysine (NBO) | 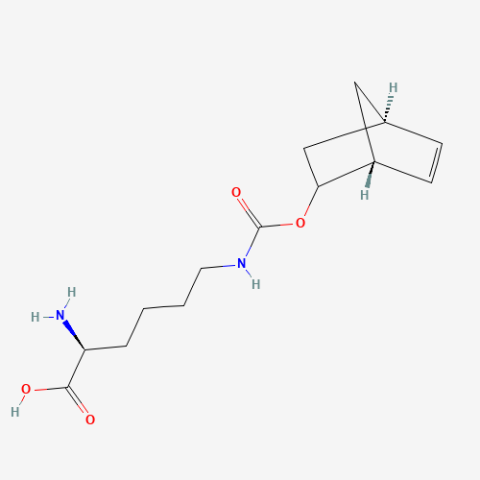
|
Reactive handle for IEDDA | Published: Fully Reviewed |
| Nε-acetyl-L-lysine | 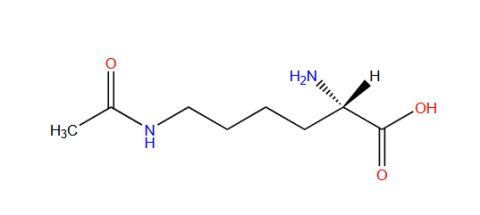
|
They are used for sites specifically studying post-translational modification acetylation events on particular lysine residues. | Published: Under Review |
| O-3-butenyl-L-tyrosine | 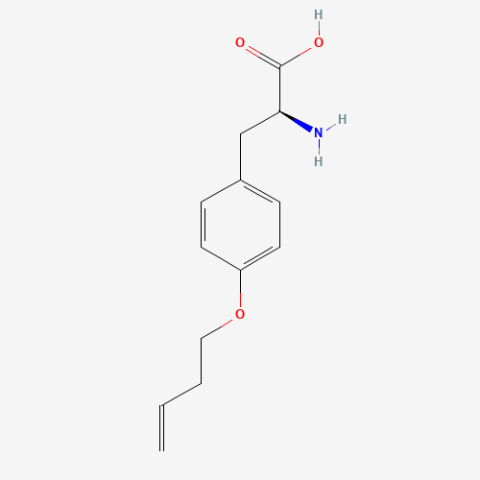
|
n/a | Published: Under Review |
| O-4-pentenyl-L-tyrosine | 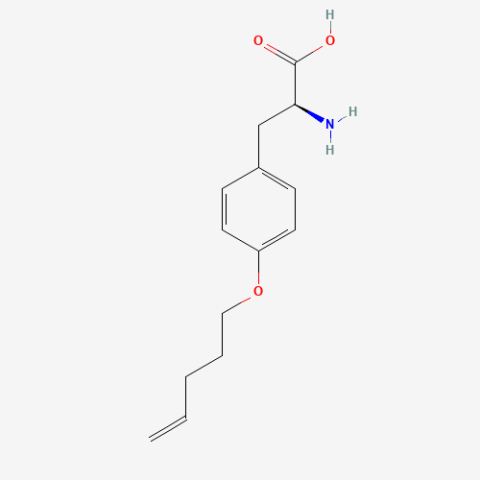
|
n/a | Published: Under Review |
| O-allyl-L-tyrosine | 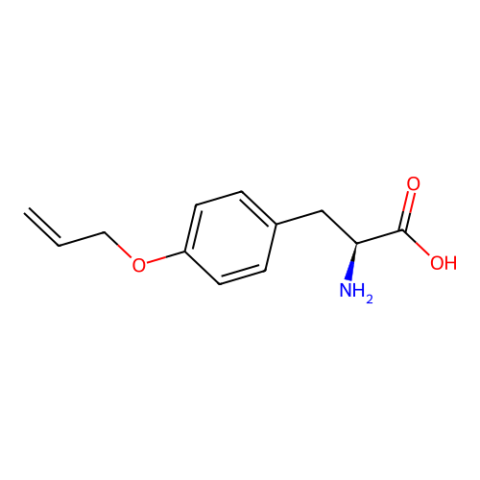
|
in E. coli expressed sfGFP2TAG at 10 mg/L using GMML media | Published: Under Review |
| O-benzyl-L-serine | 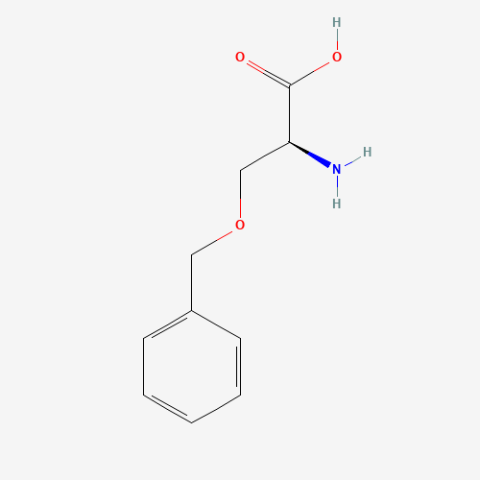
|
n/a | Published: Under Review |
| O-methyl-L-tyrosine | 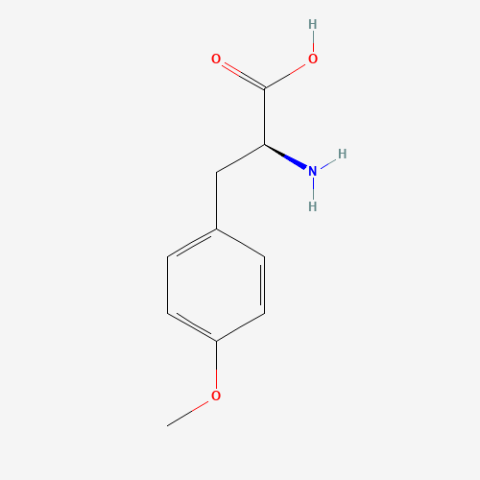
|
Labelling of muscles (global) |
Published: Under Review |
| o-nitro-L-phenylalanine | 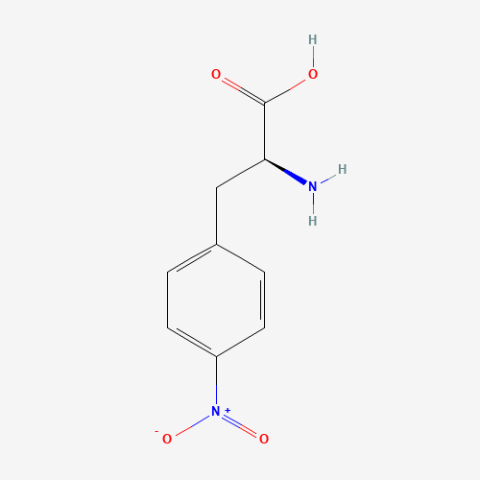
|
Bioorthogonal Ligation handle | Published: Under Review |
| O-propargyl-L-tyrosine | 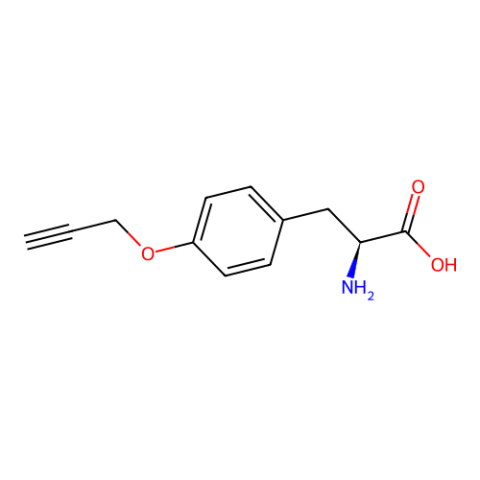
|
Reactive handle for azide-alkyne cycloaddition | Published: Under Review |
| O-tert-butyl-L-tyrosine (Tby) | 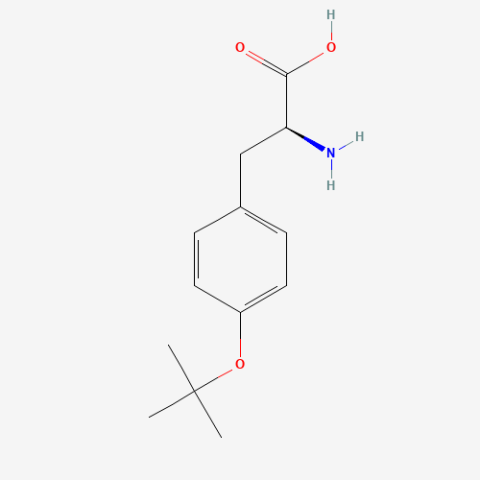
|
NMR tag for proteins | Published: Fully Reviewed |
| p-amino-L-phenylalanine | 
|
π-donating effects, hydrogen-bonding properties, and weak basicity | Published: Under Review |
| p-azido-L-phenylalanine (pAzF) | 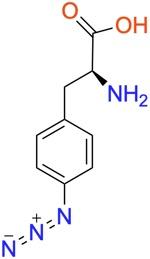
|
Used as a photocrosslinker, allowing for crosslinking and bioorthogonal click-chemistry ligation of proteins via strain-promoted cycloadditions (SPAAC) with suitably functionalized molecules. | Published: Under Review |
| p-benzoyl-L-phenylalanine (Bpa) | 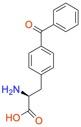
|
Used as a photocrosslinker, allowing for crosslinking and bioorthogonal ligation of protein. | Published: Fully Reviewed |
| p-boronophenylalanine | 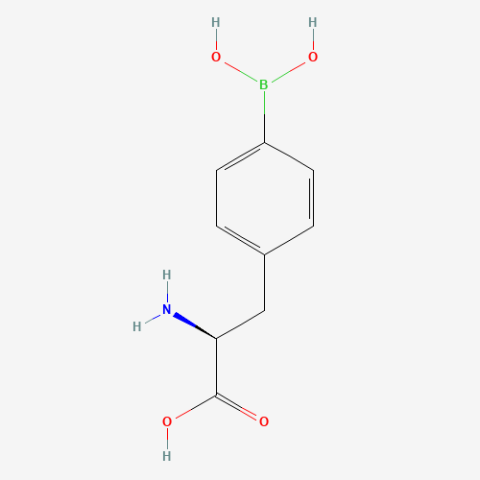
|
enabled one-step "scarless" protein purification procedure, and can be used to site-specifically label proteins using various boronic acid chemistries. Also, in second foundational paper was used in creating a fluorescent probe for peroxinitrite. | Published: Fully Reviewed |
| p-chloro-L-phenylalanine | 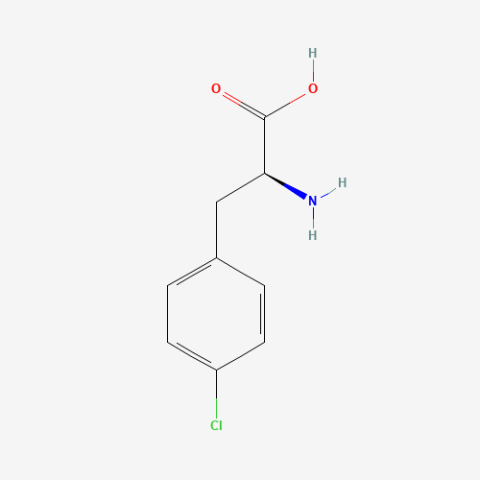
|
NMR probe | Published: Under Review |
| p-fluoro-L-phenylalanine | 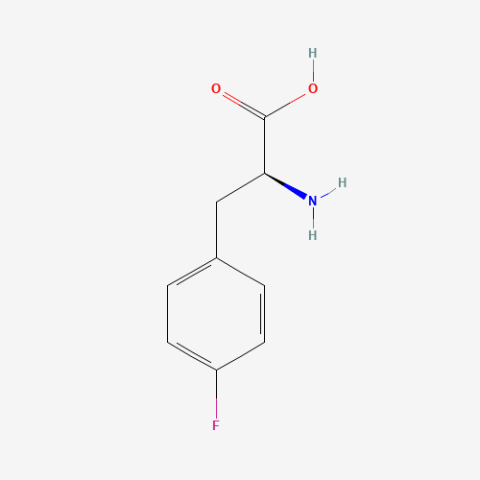
|
NMR probe | Published: Under Review |
| p-iodo-L-phenylalanine | 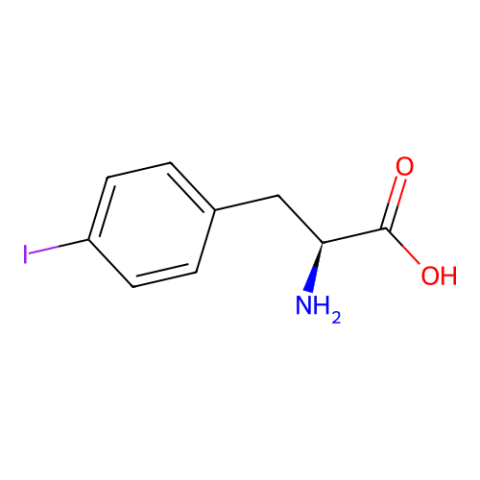
|
Can be used for phasing in protein crystallography. | Published: Under Review |
| p-trifluoroacetyl-L-phenylalanine | 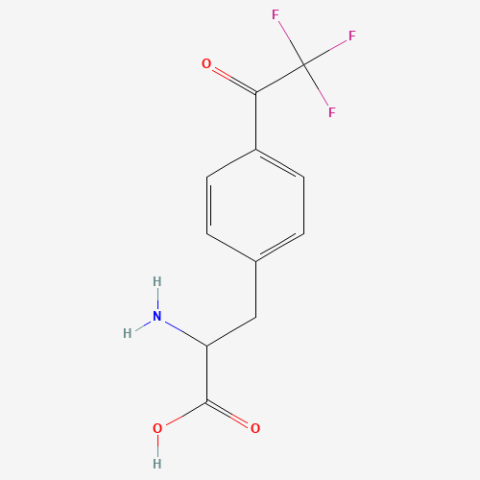
|
Flourescent Probe, Bioorthogonal ligation handle | Published: Under Review |
| phosphoserine | 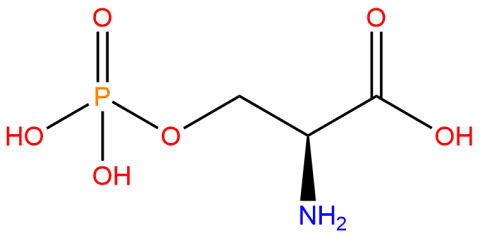
|
Used to produce proteins site-specifically phosphorylated at serine residues, typically at positions where phosphoserine naturally occurs as a post-translational modification | Published: Fully Reviewed |
| phosphothreonine | 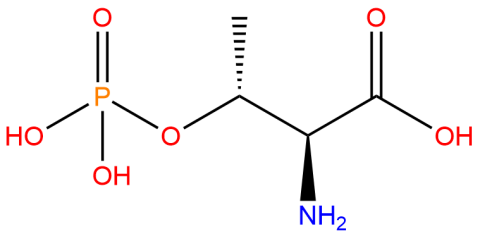
|
Used to produce site-specifically phosphorylated proteins at a threonine residue via genetic code expansion. | Published: Fully Reviewed |
| phosphotyrosine | 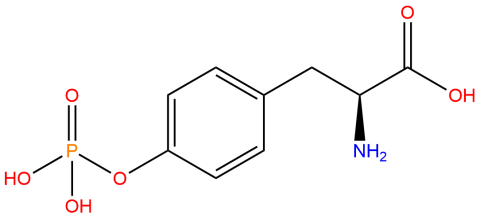
|
Used to produce site-specifically phosphorylated proteins at a tyrosine residue via genetic code expansion versus other protein phosphorylation strategies. | Published: Under Review |
| pNO2ZLys | 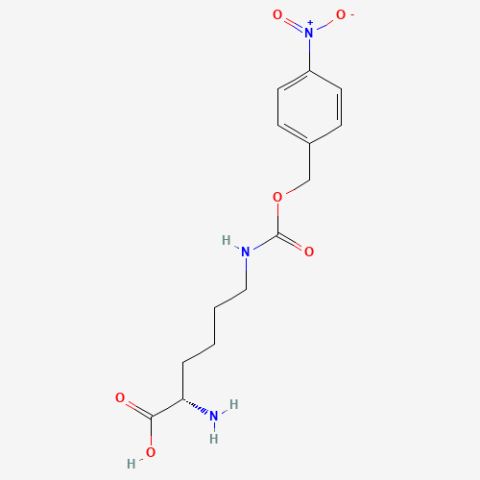
|
Photo-cross-linking agent, but no tests were done on this ncAA in the founding paper. Crosslinking experiments were only done with TmdZLys | Published: Fully Reviewed |
| propargylglycine | 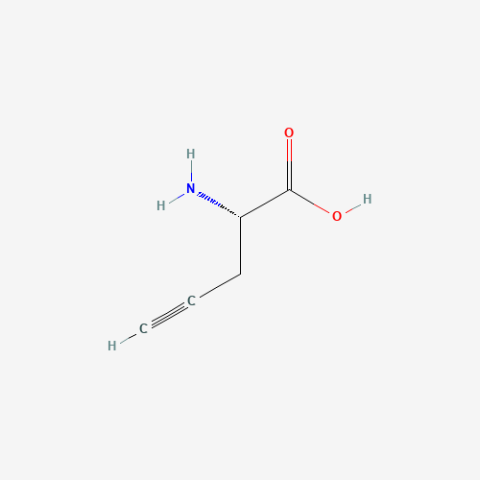
|
copper-catalyzed alkyne-azide click chemistry (CuACC) can be used for time-resolved, cell-selective proteomic analyses | Published: Fully Reviewed |
| S-Allylcysteine | 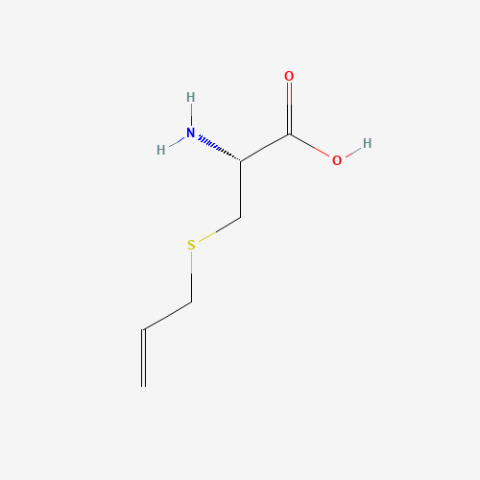
|
Reactive cross-metathesis substrate | Published: Under Review |
